Quality
Good Manufacturing Practices
In every country where we operate, we hold operating licenses and certifications for Good Manufacturing Practices and Good Distribution Practices, backed by our Quality Assurance Systems. These ensure compliance with — and often exceed — local regulatory requirements.

Our Awards and Achievements
In Mexico, the Quality Assurance System at our manufacturing site is ISO 9001 certified, It also holds an operating license and a Good Manufacturing Practices certificate issued by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS)..
This positions Stendhal as a pharmaceutical company with high global compliance standards, recognized for having:
- Market knowledge
- Cold chain storage systems
- Facilities and processes certified by COFEPRIS NOM-059-SA1-2015
- ISO 9001:2015 Certificate
- 24/7 Pharmacovigilance platform
- Business conduct and integrity

Stendhal Facilities
Our facilities focus on the manufacturing and primary packaging of oral solids, and secondary packaging of various dosage forms.
The plant is certified in Good Manufacturing Practices by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS), and also holds ISO 9001:2015 certification, which ensures not only regulatory compliance but also the proper management of a consistent and continuously improving quality system.
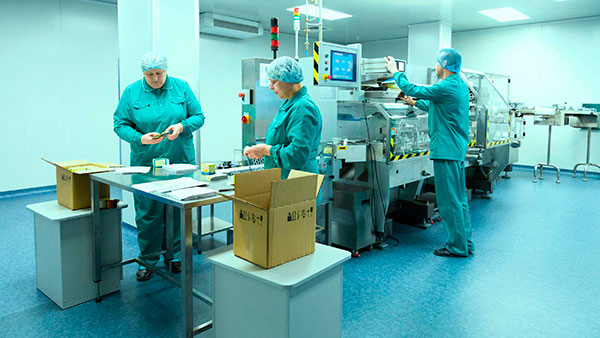
Manufacturing of secondary packaging for medication
We offer secondary packaging services for oral solids, injectables, and cold-chain medications to national and international clients in the pharmaceutical industry.
For this, we have specialized areas, equipment, processes, and qualified personnel to meet requirements with the highest quality standards and a customer service approach.

Laboratories
Our facility houses a physicochemical control laboratory for product release, stability studies, analytical transfers, validation analyses, and critical systems testing. We also have a microbiological control laboratory for testing finished products, raw materials, packaging materials, purified water, and environmental controls.
Warehouse
Our warehouse facilities support the reception, storage, picking, and shipping processes for raw materials, packaging materials, semi-finished goods, and finished products — all in regulatory compliance and managed through our ERP system (SAP)






